|

| HealthMark
|
Cleaning
Verification
Solutions
|
|
|
|
 ¹Ýº¹ÀûÀ¸·Î
¼Òµ¶
¹× ¸ê±Õ
ÈÄ »ç¿ëÇÏ´Â
Àç»ç¿ëÀÇ·á±â±â(±â±¸)ÀÇ
¹«±Õ¼º
°Ë»ç¸¦
À§ÇÑ
¼Ö·ç¼Ç.
¹Ýº¹ÀûÀ¸·Î
¼Òµ¶
¹× ¸ê±Õ
ÈÄ »ç¿ëÇÏ´Â
Àç»ç¿ëÀÇ·á±â±â(±â±¸)ÀÇ
¹«±Õ¼º
°Ë»ç¸¦
À§ÇÑ
¼Ö·ç¼Ç.
|
 ÀÎü
¹× µ¿¹°µî¿¡
»ðÀԵǴÂ
Àç»ç¿ëÀÇ·á±â±â´Â
¿©·¯
ȯÀÚ
/ °ËüÀÇ
±³Â÷»ç¿ëÀ¸·Î,
¹Ì»ý¹°»Ó
¸¸ ¾Æ´Ï¶ó
´Ü¹éÁú,
Ç÷¾×,
ÀÎü
¹× µ¿¹°µî¿¡
»ðÀԵǴÂ
Àç»ç¿ëÀÇ·á±â±â´Â
¿©·¯
ȯÀÚ
/ °ËüÀÇ
±³Â÷»ç¿ëÀ¸·Î,
¹Ì»ý¹°»Ó
¸¸ ¾Æ´Ï¶ó
´Ü¹éÁú,
Ç÷¾×,
Á¶Á÷
µî ´Ù¾çÇÑ
ÀÜ¿©¼ººÐÀ¸·Î
±³Â÷
°¨¿°µÉ
¼ö ÀÖ±â
¶§¹®¿¡
À§ÇèÀ»
ÃÖ¼ÒÈÇϱâ
À§ÇØ
öÀúÇÑ
¸ê±ÕÀÌ
ÇʼöÀûÀÔ´Ï´Ù.
|
 HealthMark
»ç Á¦Ç°Àº
¸ê±Õ
°úÁ¤ÈÄ
Àç »ç¿ëÀÇ·á
±â±¸¿¡
³²¾Æ
ÀÖÀ»
¼ö ÀÖ´Â
°¢Á¾
¿À¿°¹°ÁúÀÇ
ÀÜ·ù
»óŸ¦
È®ÀÎÇÒ
¼ö
HealthMark
»ç Á¦Ç°Àº
¸ê±Õ
°úÁ¤ÈÄ
Àç »ç¿ëÀÇ·á
±â±¸¿¡
³²¾Æ
ÀÖÀ»
¼ö ÀÖ´Â
°¢Á¾
¿À¿°¹°ÁúÀÇ
ÀÜ·ù
»óŸ¦
È®ÀÎÇÒ
¼ö
ÀÖ´Â
´Ù¾çÇÑ
method¿Í
±â±¸
¹× ÀåºñÀÇ
¼¼Ã´¿ë
Àåºñ·ùÀÇ
È¿´É
Å×½ºÆ®¿ë
methodµµ
°ø±ÞÇÕ´Ï´Ù.
|
 AAMI,
AORNÀÇ
Ç¥ÁØ
±Ô°Ý°ú
FDA,
CDCÀÇ
±ÇÀå¿¡
¸Â°Ô
±¹Á¦ÀûÀ¸·Î
À̹Ì
°ËÁõµÈ
¸ê±Õ
/ û°á
°ËÁõ
Å×½ºÆ®
¹æ¹ýÀÔ´Ï´Ù.
AAMI,
AORNÀÇ
Ç¥ÁØ
±Ô°Ý°ú
FDA,
CDCÀÇ
±ÇÀå¿¡
¸Â°Ô
±¹Á¦ÀûÀ¸·Î
À̹Ì
°ËÁõµÈ
¸ê±Õ
/ û°á
°ËÁõ
Å×½ºÆ®
¹æ¹ýÀÔ´Ï´Ù.
|
|
|
 MODELS
MODELS
|
|
ATS
2015
|
Artificial Test Soil
(Àΰø
Á¶Á¦ º¹ÇÕ¹°)
ATS
more 
|

|
Artificial
Test
Soil
is
comprised
of
the
organic
contaminants
remaining
on
surgical
instruments
after
clinical
use,
including
protein
(~23mg/ml),
hemoglobin
(~8mg/ml)
and
carbohydrates
(~6mg/ml).
These
quantities
are
reflective
of
the
¡°worst
case¡±
levels
found
to
remain
on
instruments
after
patient
use,
but
before
reprocessing.
The
FDA
and
other
international
regulatory
agencies
specify
that
simulated-use
testing
should
approximate
as
closely
as
possible
the
actual
soiling
the
instrument is
exposed
to
during
clinical
use.
ATS
meets
this
requirement
with
extensive
research
(available
upon
request)
to
support
it.
|
|
ATS2015
Instruction
|

|
|
ATS2015
MSDS
|

|
|
|
Cat.
no.
|
Description
|
|
ATS2015
- 1ML
|
1ml
Soil
Test
|
|
ATS2015-
9ML
|
9ml
Soil
Test
|
|
ATS2015-
100ML
|
100ml
Soil
Test
|
|
ATS2015-
500ML
|
500ml
Soil
Test
|
|
BTS
|
Blood Test Soil
(Àΰø
Á¶Á¦ º¹ÇÕ
Ç÷¾×)
|

|
Quick,
easy
and
affordable
Blood
Test
Soil
(BTS)
is
a
simple,
simulated
blood
soil
that
visually
mimics
blood
on
the
exterior
of
patient-used
instruments.
The soil is primarily comprised of only blood proteins: bovine albumin and
hemoglobin in known quantities and is a reproducible formulation.
The soil also contains sodium alginate and calcium chloride solution that
cross link to form alginate hydrogels.
These 3-D protein hydrogels simulate the fibrin hydrogels in clotted blood.
It
has
a
long,
stable
shelf
life
in
its
lyophilized
form.
Just
rehydrate
with
sterile
water
and
it
is
ready
to
use.
|
|
BTS
Instruction
|

|
|
BT5
MSDS
|

|
|
|
Cat.
no.
|
Description
|
|
BTS-
7ML
|
7ml
Soil
Test
|
|
BTS-
100ML
|
100ml
Soil
Test
|
|
BTS5-
500ML
|
500ml
Soil
Test
|
|
DTS
|
Dental Surgical Test Soil
(Ä¡°ú¿ë
Àΰø
Á¶Á¦ º¹ÇÕ¹°
)
|
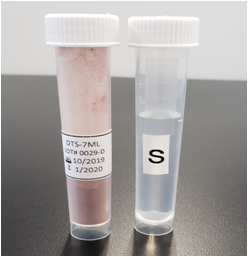
|
Dental Surgical Test Soil (DTS) is a reproducible formulation with blood
proteins, artificial saliva, mucus and bone particulates. It is representative
of soil found on patient used instruments after dental surgical procedures.
|
|
DTS
Instruction
|

|
|
DT5
MSDS
|
|
|
|
Cat.
no.
|
Description
|
|
DTS-
7ML
|
7ml
Soil
Test
|
|
DTS-
100ML
|
100ml
Soil
Test
|
|
HEMOCHECK
|
Hemoglobin residue on
surfaces ±â±¸ Ç¥¸é»óÀÇ ÀÜ·ù Hemoglobin (1ug) °ËÃâ
in 30 seconds
|

|
The HemoCheck¢â is an all-in-one test, provides a result in 30 seconds, is
simple to interpret and indicates blood residue down to 0.1¥ìg.
Significant traces of blood indicate an inadequately reprocessed instrument
and is a rich source for cross contamination.
To use the HemoCheck¢â, simply swipe the surface of the instrument, drop the
swab in the activated indicator, shake vigorously, wait for 30 seconds and
check for color change. If any area of the swab turns green or blue, the surface
harbors blood residue and should be reprocessed.
If many instruments are found to be soiled, a thorough evaluation of the
decontamination processes should be conducted. HemoCheck¢â complies with
ASTM Guide D7225.
|
|
HemoCheck
Instruction
|

|
|
HemoCheck
MSDS
|
|
|
|
Cat.
no.
|
Description
|
|
HC-101
|
Hemocheck
Blood
Detection
Tests
(12
tests
/ pk)
|
|
PROCHECK
II
|
Protein residue on
surfaces
±â±¸ Ç¥¸é»óÀÇ ÀÜ·ù Protein (1ug) °ËÃâ
in 30 seconds
|

|
The ProChek-II¢â is simple to use, provides a rapid result, is an all-in-one test,
and is easy to interpret. The ProChek-II¢â measures for residual protein on
surfaces. Protein is a significant component of bioburden.
Traces of protein indicate an unclean surface and a likely source of cross
contamination. To use the ProChek-II¢â, moisten the tip with sterile water,
swab the surface of the item being tested, drop the swab in the vial and
shake vigorously. If the color of the liquid remains brown, the surface is
demonstrably clean. If the liquid or any part of swab turns blue-green, the
surface harbors protein and should be re-cleaned.
The test is sensitive to 1 µg of protein.
|
|
ProCheck
Instruction
|

|
|
ProCheck
MSDS
|
|
|
|
Cat.
no.
|
Description
|
|
PT-202
|
Procehck
Protein
Detection
Tests
(12
tests
/ pk)
|
|
PROCHECK
W
|
Protein residue on
surfaces
±â±¸ Ç¥¸é»óÀÇ ÀÜ·ù Protein (1ug) °ËÃâ
in 30 seconds
|

|
ProCheck-W (PT-Q-002) is a quantitative protein test to determine the
amount of residual protein remaining in lumened instruments after
postprocedure
cleaning prior to
further processing.
The
Test is an easy
to use and interpret
method, which provides
rapid
results
and comes with the
following: 15 Protein
Reagent Vials, 15
Blue Vial
Screw
Caps, 15 Pipettes,
15 Ziploc Bags,
and an Interpretation
Color Chart.
The test can detect and quantify proteins in a range of 1µg - 30µg ± 1µg, showing
the readings on the spectrophotometer.
|
|
ProCheckW
Instruction
|

|
|
ProCheckW
MSDS
|
|
|
|
Cat.
no.
|
Description
|
|
WPT-Q-002
|
Quantitive
Protein
Detection
Tests
(15
tests
/ pk)
|
|
HYDROCHECK
|
Moisture
residue on
surfaces
±â±¸ Ç¥¸é»óÀÇ ÀÜ·ù moisture (0.05uL) °ËÃâ
|

|
Designed for detecting residual moisture in channels, the single-use
HydroCheck¢â assists in detecting moisture.
The user-friendly test kit can detect as little as 0.05 ¥ìL of residual moisture,
providing immediate results. Swabs are available in the following
sizes: 1.7mm, 2.8mm, 3.8mm, and 5.0mm. If a detectable amount of residual
moisture is present on the swab, there will be a visual color change to
purple on the swab.
For
periodic quality
assurance testing
of endoscopes. Endoscopes
should
be
reprocessed after
testing.
|
|
Hydrocheck
Instruction
|

|
|
Hydrocheck
MSDS
|
|
|
|
HCat.
no.
|
Description
|
|
HYD-200
|
Hydrocheck
: 1.7mm
|
|
HYD-270
|
Hydrocheck
: 2.7mm
|
|
HYD-350
|
Hydrocheck
: 3.8mm
|
|
HYD-470
|
Hydrocheck
: 5.0mm
(15
tests
/ pk)
|
|
TOSI
|
SoilÀ»
ÀÌ¿ëÇÏ¿© ±â±¸ ¼¼Ã´ ÀåºñÀÇ
¼¼Ã´´É
°ËÁõ.
|

|
TOSI® is a
consistent, repeatable, and reliable method for evaluating the
cleaning effectiveness of the automated instrument washer.
When metered on to the stainless steel plate, the TOSI® is completely
analogous to a stainless steel instrument soiled with dried blood.
Placed in the see-through plastic holder, the challenge is identical to the
areas of instruments typically hidden from view (i.e., box locks).
The routine use of this test will help insure that your instrument washer is
performing at a consistent level, enhancing the routine
visual inspection of
instruments. TOSI® complies with new AAMI and AORN
Guidelines as well as ASTM
Guide D7225.
|
|
TOSI
Instruction
|

|
|
TOSI
MSDS
|
|
|
|
Cat.
no.
|
Description
|
|
WT-101
|
Proformance
Washer
Test
(30
tests
/ pk)
|
|
|
|
|